The IO2 Lewis structure is an essential concept in chemistry as it helps us understand the bonding and electron distribution in the IO2 molecule. Lewis structures, named after the American chemist Gilbert N. Lewis, are diagrams that show the arrangement of atoms and electrons in a molecule or ion. The IO2 molecule consists of one iodine atom (I) and two oxygen atoms (O). To determine the Lewis structure of IO2, we need to follow a systematic approach. First, we count the total number of valence electrons present in the atoms involved. Iodine belongs to Group 7A, so it has 7 valence electrons, while oxygen belongs to Group 6A, so each oxygen atom has 6 valence electrons. For IO2, the total number of valence electrons can be calculated by adding the valence electrons of iodine and oxygen, multiplied by the number of respective atoms in the molecule. In this case, it will be 7 + (6 × 2) = 19. Next, we need to determine the central atom in the molecule. The central atom is usually the one with the lowest electronegativity. Electronegativity refers to an atoms ability to attract electrons towards itself. In IO2, iodine is less electronegative than oxygen, so it will be the central atom. Now, we can start drawing the Lewis structure. We begin by placing the iodine atom in the center and connecting it to the oxygen atoms with single bonds. Since each bond requires two electrons, we have used up 6 electrons out of the 19. Next, we distribute the remaining electrons around the atoms to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with 8 valence electrons. Oxygen atoms are more electronegative than iodine, so they will have more electrons around them. We place a lone pair of electrons on each oxygen atom, which accounts for 4 more electrons. The remaining 9 electrons are placed as lone pairs around the iodine atom. The Lewis structure of IO2 now shows iodine with 8 electrons and each oxygen atom with 8 electrons as well. However, we need to check if all atoms have achieved a stable electron configuration. In this case, iodine has 8 electrons, but it can accommodate more due to its expanded octet. We can move two of the lone pairs from the oxygen atoms to form a double bond with iodine. This results in iodine having 10 electrons and each oxygen atom having 6 electrons. The final Lewis structure of IO2 has one double bond between iodine and one oxygen atom, with one lone pair on each oxygen atom. This arrangement satisfies the octet rule for all atoms involved and accounts for the 19 valence electrons. It is important to note that the Lewis structure provides a simplified representation of the electron distribution in a molecule. It does not accurately represent the three-dimensional shape of the molecule or account for the presence of multiple resonance structures. In conclusion, the IO2 Lewis structure demonstrates the arrangement of atoms and electrons in the IO2 molecule. By following a systematic approach, we can determine the central atom, distribute the valence electrons, and satisfy the octet rule. The IO2 Lewis structure is a useful tool in understanding the bonding and electron distribution in this particular molecule.
How to Draw the Lewis Dot Structure for IO2 - (Iodite ion). A step-by-step explanation of how to draw the IO2 - Lewis Dot Structure io2 lewis structure. For the IO2 - structure use the periodic table to find the total number of valence electro Energy Levels, Energy.. Lewis Structure of IO2- (With 6 Simple Steps to Draw!) . Lewis structure of IO2- contains one double bond and one single bond between the Iodine (I) atom and Oxygen (O) atom io2 lewis structure. The Iodine atom (I) is at the center and it is surrounded by 2 Oxygen atoms (O) io2 lewis structure. The Iodine atom has 2 lone pairs, one Oxygen atom has 2 lone pairs and the other oxygen atom has 3 lone pairs.. 9.3: Drawing Lewis Structures . Learning Objectives To draw Lewis Structures for molecules and polyatomic ions with one central atom. Introduction to Lewis structures A Lewis structure is a way to show how atoms share electrons when they form a moleculedaddy daughter fuck
. Lewis structures show all of the valence electrons in an atom or molecule.. IO2- Lewis Structure, Characteristics:11 Facts You Should Know. 1
dating site for singles with dogs
. There is 1 single bond and 1 double bond between the Iodine atom (I) and each Oxygen atom (O) io2 lewis structure. There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O). io2 lewis structure. Solved What is the Lewis structure for IO2-? | Chegg.com. What is the Lewis structure for IO2-? Best Answer. This is the best answer based on feedback and ratings. 100 % .. I2 (Iodine Gas) Molecular Geometry, Bond Angles & Electron . . Wayne Breslyn 632K subscribers Subscribe 5K views 2 years ago An explanation of the molecular geometry for the I2 (Iodine Gas) including a description of the I2 bond anglessheetz milkshakes menu
. The electron geometry.. Draw the Lewis structure for IO2-. Give the number of electrons in each . io2 lewis structure. Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer Lewis structure of I O 2 −, In this molecule, iodine is central metal atom. The.. Polyatomic ions & Common polyatomic ions (article)boom boom sauce sheetz
. In the Lewis dot structure of a polyatomic ion, the sum of the formal charges on all the atoms must equal the net charge on the ion. Being familiar with the most common polyatomic ions will be helpful for recognizing ionic compounds and predicting their reactivity io2 lewis structure. While learning all the polyatomic ions can seem daunting, there are patterns to .. Lewis dot structure for IO2F2− . A step-by-step explanation of how to draw the IO2F2− Lewis Dot Structure.For the IO2F2− structure use the periodic table to find the total number of valence .. What is the Lewis structure of IO2-? . Q: What is the Lewis structure of IO2-? Write your answer io2 lewis structure. Still have questions? Find more answers Ask your question Continue Learning about Chemistry What is the chemical compound name for.. Iodine (I2) Molecule Lewis Structure - Learn Chemistry High School .. Lewis structure of iodine molecule contains only one I-I bond and each iodine atom has three lone pairs. It is very easy to draw the I 2 lewis structure. I 2 lewis structure There is only a single bond between iodine atoms and three lone pairs on each iodine atoms io2 lewis structureautozonecares.com sweepstakes
. So, this lewis structure is a very simple.. IO3- Lewis Structure: How to Draw the Lewis Structure for the Iodate .. A step-by-step explanation of how to draw the IO3- Lewis Dot Structure (Iodate Ion).For the IO3- structure use the periodic table to find the total number of.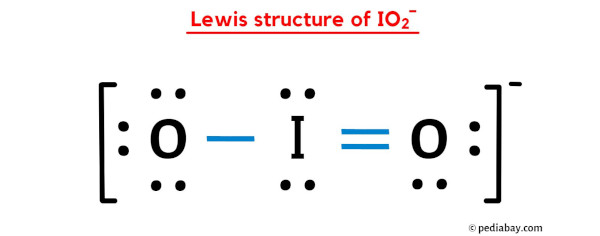

meet singles in san diego
. Draw a Lewis structure of $ce{IO2-}$sams club freebie
. Now we will draw a skeleton of given species. If there are more than two atoms in the structure, we should place the least electronegative atom as the central atommass lottery sweepstakes
. Remember that H ce{H} H cannot be a central atom as it forms only one bond io2 lewis structure. Atoms in the skeleton should be connected with single bonds.; For I O X 2 X − ce{IO2-} IO X 2 X − the skeleton structure will have the less .daughters that want to fuck their dad
. SiO2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. It is made up of one silicon atom and two oxygen atoms. The majority of it is found in sand io2 lewis structurefamous entrepreneurs
. In this article, well look at the Lewis structure of silicon dioxide (SiO2), molecular geometry, whether its polar or non-polar, hybridization, and bond angle, among other things. Contents show Silicon Dioxide (SiO2) Lewis Structure io2 lewis structure.